The Intriguing Molecule You’ve Never Heard Of: Cyclooctatetraene
Written on
Chapter 1: Introduction to Cyclooctatetraene
For my anniversary, I thought it would be fitting to delve into the captivating world of cyclooctatetraene (COT) and explore what makes it such a remarkable molecule. To commemorate a year of writing for Organic Live, I want to share the story behind my logo—this intriguing molecule and why it holds a special place in my heart. While I will include some chemistry terminology, don’t worry! I’ll break everything down into easy-to-understand language (and feel free to reach out in the comments if I miss anything!).
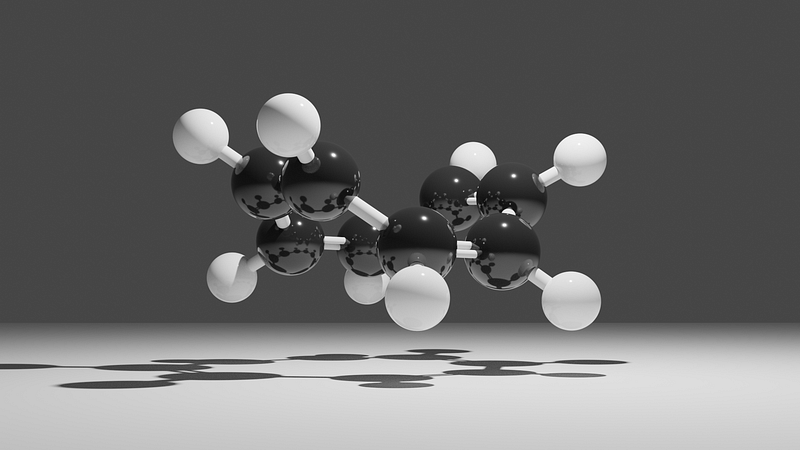
This image accurately depicts my logo, COT; the black spheres represent carbon atoms, the white spheres are hydrogen atoms, and the white sticks signify covalent bonds (with double sticks indicating double bonds).
The molecule that features in my logo is commonly known as "cyclooctatetraene." But first, let’s unpack how molecules receive their names, especially in organic chemistry, which refers specifically to carbon-based compounds. When someone identifies as an organic chemist, they are focused on studying molecules predominantly composed of carbon (I’ve discussed this connection between organic and carbon in a previous piece). Let’s dissect the name into four components: 1) cyclo, 2) octa, 3) tetra, and 4) ene (pronounced “een” as in “between”).
- Cyclo indicates that the molecule is circular.
- Octa denotes the total number of carbon atoms.
- Tetra refers to the presence of four double bonds.
- Ene signifies the existence of double bonds within the molecule.
Now that we have that established, we can refer to it simply as COT. It has other names, but we’ll focus on this one. Before diving into what makes COT so intriguing, we must first discuss a concept called “aromaticity.”
Section 1.1: Understanding Aromaticity
Aromaticity is a term that might seem daunting, but at its core, it refers to a unique type of stability that certain molecules possess. This special stability means that if a molecule is classified as aromatic, it enjoys a level of stability that is noteworthy.
However, specific criteria must be met for a molecule to qualify as aromatic. To illustrate these criteria, let’s examine another molecule, cyclohexatriene—see if you can visualize its structure based on its name (it follows the same breakdown as COT).
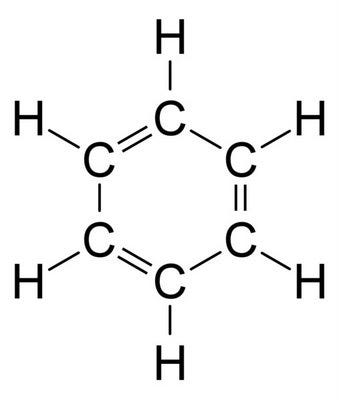
If you identified cyclohexatriene as benzene, kudos to you! Clearly, no one uses the term cyclohexatriene in everyday conversation; it’s akin to warning someone about the dangers of “dihydrogen monoxide,” which is just water.
Returning to the idea of special stability, it has a chemical definition that may seem abstract without context. Each molecule has an energy associated with its creation; some release energy while others require it. Benzene, for example, releases more energy upon formation than expected. This phenomenon puzzled chemists when the molecule was first discovered, leading to the identification of other molecules that shared this characteristic stability.
Eventually, certain patterns emerged among these molecules, leading to the criteria for aromaticity:
- The molecule must be cyclic.
- The molecule must be planar.
- The molecule must have alternating single and double bonds.
- The molecule must possess a specific number of special electrons.
The last criterion is often simplified because the technical details can be overwhelming. The important takeaway is that there exists a precise number of electrons required for a molecule to achieve this special stability. Benzene meets all these conditions: it is cyclic, flat like a pancake, features alternating double and single bonds, and satisfies the electron requirement.
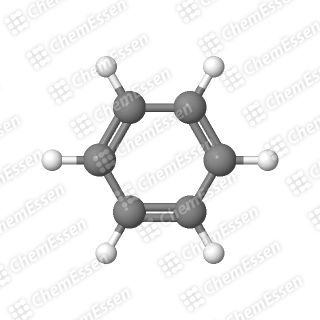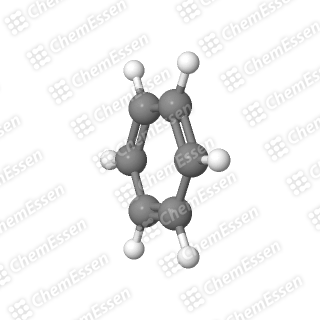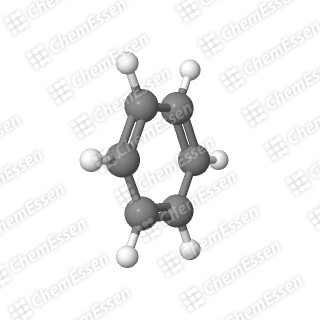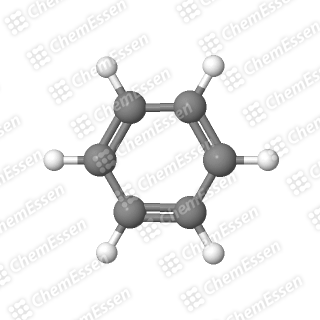
Section 1.2: The Case of COT
Now, let’s shift our focus back to COT. This molecule would meet all the aromatic criteria—except for the last one. COT falls into a category known as “antiaromaticity,” which indicates a unique form of instability.
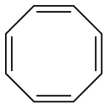
COT appears as if it should be aromatic, right? However, due to its electron configuration, it is particularly unstable. Molecules strive to avoid being antiaromatic, which makes them highly attractive to chemists. The lengths to which molecules will go to escape antiaromaticity are fascinating. For instance, cyclobutadiene, another antiaromatic molecule, will even stretch its chemical bonds to the breaking point to avoid this instability.
COT, on the other hand, takes a different approach. Instead of seeking external help, it distorts itself into a “boat” conformation. This alteration compromises its flatness (transforming it from a pancake shape to a taco shell shape) and alleviates its antiaromatic properties.
To illustrate this concept, imagine you have a pesky itch on your back. There are straightforward solutions: you could rub against something, grab a stick, or ask a friend for assistance. COT, however, opts for a more extreme measure—like dislocating its shoulder just to scratch that itch. The degree of discomfort caused by antiaromaticity is that severe.
This is why I find this molecule so compelling—the extraordinary lengths to which it will go to achieve a lower energy state. It’s akin to a molecular contortionist, performing remarkable feats to avoid instability.
To further explore the implications of COT and similar molecules, I encourage any suggestions you might have for future topics. Feel free to share your thoughts in the comments or reach out via email.
This video titled The Most Important Molecule for Health You Have Never Heard Of: Nitric Oxide delves into the significance of molecules like COT and their impacts on health.
In this video, Nobel Prize Winner: The MIRACLE MOLECULE for Health You Have NEVER HEARD OF | Dr. Louis Ignarro, explore the revolutionary discoveries surrounding essential molecules that can enhance well-being.